MoonLake Receives FDA Feedback on SLK Clinical Evidence Strategy for Hidradenitis Suppurativa
- Clinical Trial Results: MoonLake's SLK treatment demonstrated significant improvements in over 1,000 HS patients across the MIRA, VELA-1, and VELA-2 trials, with a 43% response rate at 120mg SLK in MIRA, showing a 29-point delta versus placebo (p < 0.001), establishing a strong market potential for SLK.
- Positive FDA Feedback: The FDA indicated that MoonLake could establish substantial evidence of SLK's effectiveness without additional clinical trials, which not only accelerates the BLA submission process but also potentially reduces future R&D costs, enhancing the company's position in the competitive biopharmaceutical market.
- Market Application Preparation: MoonLake is on track to submit its BLA in the second half of 2026, with FDA advising the inclusion of VELA-2 trial results to assess SLK's safety, paving the way for product launch and further increasing the company's market share in dermatological treatments.
- Potential Acquisition Opportunity: In June 2025, Merck approached MoonLake with a bid exceeding $3 billion, reflecting high market recognition of SLK's potential value, which could provide additional funding support for MoonLake to accelerate its R&D and market promotion.
Trade with 70% Backtested Accuracy
Analyst Views on MLTX
About MLTX
About the author

- New Chief Medical Officer: ImageneBio has appointed Dr. Ben Porter-Brown as Chief Medical Officer, who brings over 20 years of clinical development experience in autoimmune and inflammatory diseases, focusing on executing the Phase 2b ADAPTIVE trial to enhance the company's expertise in this area.
- International Trial Expansion: Dr. Porter-Brown plans to expand the Phase 2b trial footprint with international sites in the UK and Europe, which will not only aid in patient recruitment but also enhance Imagene's competitiveness in the global market.
- Innovative Treatment Potential: Imagene's IMG-007 is a monoclonal antibody targeting OX40 with unique product features, expected to provide new treatment options for patients with moderate-to-severe atopic dermatitis, filling a market gap and driving future growth for the company.
- Equity Incentive Plan: As an inducement for joining, Imagene will grant Dr. Porter-Brown 137,000 shares of common stock, reflecting the company's commitment to his leadership and aiming to attract top talent to drive the success of clinical projects.
Biotech Sector Growth: The biotech sector, particularly Moon Lake Immunotherapeutics, is gaining attention amid interest rate cuts and an AI boom, with significant stock price increases and a focus on clinical-stage biotech investments.
FDA Fast Track Designation: Moon Lake's lead drug, Sonelokimab, received Fast Track designation from the FDA for treating Palmoplantar Pustulosis, addressing a significant unmet medical need in the U.S. market.
Financial Stability: The company holds approximately $380.5 million in cash and equivalents, providing a strong financial foundation to support its regulatory filings and potential commercialization efforts without immediate capital raises.
Upcoming Investor Day: Moon Lake is set to host an Investor Day on February 23, 2026, focusing on data from its Phase 2 SOLARIS trial, which could further validate its drug's efficacy across various inflammatory diseases.
- Clinical Trial Progress: Avalo Therapeutics has completed enrollment for the Phase 2 LOTUS trial of AVTX-009, involving approximately 250 adults with moderate to severe hidradenitis suppurativa, aimed at evaluating the efficacy and safety of subcutaneous bi-weekly and monthly dosing regimens, with topline data expected in mid-2026.
- Stock Volatility Risk: Analyst Cantor highlights that if the stock underperforms, it could decline by 85-90%, while Agarwal sees potential upside even if the HiSCR75 change is around 18%-19%, though the Phase 3 trial poses risks that may dampen investor confidence.
- Potential Upside Forecast: Should the HiSCR75 change fall between 20-25%, Avalo's stock could rally by 100-150%, and if it drops to 25-30%, the stock might skyrocket over 200%, indicating the market's sensitivity to efficacy outcomes.
- Analyst Rating Trends: Recently, Avalo Therapeutics has seen a positive trend in analyst ratings, with Guggenheim and HC Wainwright & Co. both issuing 'Buy' ratings, with Guggenheim setting a target price of $50.00, reflecting a bullish outlook for the company.
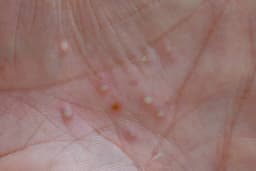
- Fast Track Designation: The US FDA has granted Fast Track designation for MoonLake Immunotherapeutics' sonelokimab (SLK) for treating moderate-to-severe palmoplantar pustulosis, a rare inflammatory disorder, indicating FDA's recognition of the drug's potential.
- Clinical Trial Support: This decision is based on results from the ongoing phase 2 LEDA trial, suggesting that sonelokimab shows promising efficacy in clinical trials, potentially offering new treatment options for patients.
- Accelerated Development: CEO Jorge Santos da Silva stated that this designation “can support the acceleration of our phase 3 program,” which will help shorten the time to market and enhance the company's competitiveness in the market.
- Positive Market Reaction: Following the FDA's Fast Track designation, MoonLake's stock has shown strong upward momentum, reflecting investor optimism regarding the drug's future success.
- Fast Track Designation: The FDA has granted Fast Track designation to sonelokimab for the treatment of moderate-to-severe palmoplantar pustulosis (PPP), based on positive results from the Phase 2 LEDA trial, which is expected to expedite the subsequent clinical development process.
- Clinical Trial Plans: The company plans to submit a Biologics License Application (BLA) in H2 2026 and will discuss the latest developments in the PPP program at the upcoming Investor Day, showcasing its strategic positioning in dermatology.
- Market Opportunity: PPP is a severe chronic inflammatory disease with no approved treatments, and the FDA's Fast Track designation will help address this significant unmet medical need, indicating a substantial market potential.
- Future Milestones: MoonLake anticipates releasing multiple key clinical data points in 2026, including the Phase 3 trials for PPP and progress in other indications, further solidifying its leadership position in the biopharmaceutical sector.
- Chipmaker Rally: TSMC's forecast of stronger-than-expected Q1 sales and an increase in 2026 capex from $40.9B to $52B-$56B has boosted chipmaker stocks, enhancing market confidence in the sustainability of AI demand.
- Strong US Economic Data: Weekly initial jobless claims unexpectedly fell by 9,000 to 198,000, indicating a stronger labor market than the anticipated rise to 215,000, further supporting stock market gains.
- Hawkish Fed Comments Impact: Atlanta Fed President indicated the need for a restrictive monetary policy due to ongoing inflation pressures, causing the 10-year T-note yield to rise by 3 basis points to 4.16%, which exerted some pressure on the stock market.
- Mixed International Market Performance: The Euro Stoxx 50 closed up by 0.60%, while China's Shanghai Composite fell by 0.33%, reflecting a divergence in global market trends, prompting investors to focus on upcoming economic data.







