Kuehn Law Encourages Investors of Actinium Pharmaceuticals, Inc. to Contact Law Firm
Investigation of Actinium Pharmaceuticals: Kuehn Law, a shareholder litigation firm, is investigating potential breaches of fiduciary duties by officers and directors of Actinium Pharmaceuticals related to misleading statements about the company's drug approval process and trial data.
Call for Shareholder Action: Shareholders who purchased ATNM stock before October 31, 2022, are encouraged to contact Kuehn Law to enforce their rights, as there may be limited time to participate in the legal proceedings.
Trade with 70% Backtested Accuracy
Analyst Views on ATNM
About ATNM
About the author

- Investor Attention: As the earnings season unfolds, mid to low market capitalization healthcare stocks are drawing investor attention due to their strong earnings momentum, indicating growing market confidence in this sector.
- Analyst Expectations: The EPS Revision Grade reflects the trend in analyst earnings estimates, with A+ ratings indicating optimistic projections for future performance, potentially driving stock prices higher.
- List of A+ Rated Stocks: Currently, companies such as Aldeyra Therapeutics, Altimmune, Annovis Bio, and Assertio Holdings have received A+ EPS Revision Grades, showcasing their strong performance in the eyes of analysts.
- Market Strategy Impact: These A+ rated healthcare stocks are likely to attract more investor interest, potentially triggering positive sentiment towards the healthcare sector as a whole, thereby enhancing the performance of related ETFs.
- FibroBiologics Outperformance: FibroBiologics, Inc. (FBLG) surged 7.68% in after-hours trading to close at $0.41, indicating speculative interest or technical momentum despite no specific news.
- Nyxoah Earnings Boost Confidence: Nyxoah SA (NYXH) advanced 3.94% to $5.28 after reporting preliminary Q4 and full-year 2025 results, with guidance for Q1 2026 enhancing investor confidence in its growth trajectory.
- Fortress Biotech's Continued Volatility: Fortress Biotech, Inc. (FBIO) climbed 6.90% to $4.49 in after-hours trading, continuing a trend of volatility without any fresh news impacting the stock.
- Revvity Collaboration Drives Growth: Revvity, Inc. (RVTY) posted a 4.92% gain to close at $109.00, as investors digest the January announcement of a collaboration with Eli Lilly to expand access to predictive models via the Revvity Signals platform.
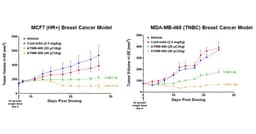
- Strong Anti-Tumor Activity: ATNM-400 demonstrated significant tumor growth inhibition across various breast cancer models, particularly in hormone receptor-positive and triple-negative cases, with all treatment regimens well tolerated and no significant body weight changes observed, indicating its potential clinical value.
- Effectiveness Against Resistant Tumors: The drug induced irreversible DNA damage in standard treatment-resistant breast cancer models, showcasing its unique advantage in addressing resistant tumors and potentially offering new treatment options for patients.
- Broad Applicability: ATNM-400 not only shows strong efficacy in breast cancer but also exhibits promising anti-tumor activity in prostate cancer and non-small cell lung cancer, supporting its potential as a targeted radiotherapy for multiple solid tumors.
- Market Demand Alignment: With an estimated increase to 250,000 women living with metastatic breast cancer by 2030, the development of ATNM-400 aligns perfectly with this growing market need, potentially providing new hope for patients with hard-to-treat breast cancer.
- Potent Anti-Tumor Activity: ATNM-400 demonstrated significant tumor growth inhibition across various breast cancer models, particularly in hormone receptor-positive and triple-negative types, with all treatment regimens well tolerated and no significant weight changes observed, indicating its potential for clinical application.
- Efficacy in Resistant Models: In trastuzumab- and tamoxifen-resistant breast cancer cells, ATNM-400 exhibited enhanced cytotoxicity, and combining it with standard treatments resulted in greater cytotoxicity and tumor regression, showcasing its promise as a combination therapy.
- Mechanistic Evidence of DNA Damage: Treatment with ATNM-400 led to irreversible double-strand DNA breaks in breast cancer cells, activating AKT phosphorylation, which indicates its effective mechanism in resistant models and may provide new options for treating hard-to-treat breast cancer.
- Broad Applicability: Beyond breast cancer, ATNM-400 has shown potential efficacy in prostate cancer and non-small cell lung cancer, supporting its development as a multi-indication targeted radiotherapy to meet clinical needs.

New Treatment for Resistant Cancers: Actinium Pharmaceuticals announced promising preclinical data for ATNM-400, an antibody radioconjugate showing anti-tumor activity in hormone-resistant and HER2-resistant breast cancer, as well as efficacy in prostate and non-small cell lung cancers.
Presentation at SABCS: The data will be presented at the 2025 San Antonio Breast Cancer Symposium, highlighting ATNM-400's potential to overcome resistance to standard therapies like tamoxifen and trastuzumab, and its ability to synergize with existing treatments.
Broad Indication Potential: ATNM-400 demonstrates best-in-class efficacy across multiple solid tumors, with significant opportunities in high-value markets, including prostate cancer and NSCLC, where it has shown superior activity compared to leading therapies.
Innovative Mechanism: Utilizing Actinium-225, ATNM-400 targets a disease-driving protein linked to poor prognosis, offering a novel approach that may improve outcomes for patients with difficult-to-treat cancers, addressing significant unmet clinical needs.

ATNM-400 Overview: Actinium Pharmaceuticals' ATNM-400 is a first-in-class antibody radioconjugate targeting a non-PSMA antigen, demonstrating potent anti-tumor activity and prolonged survival in prostate cancer models resistant to standard therapies like enzalutamide and 177Lu-PSMA-617.
Efficacy and Resistance: Preclinical studies show that ATNM-400 outperforms existing treatments, achieving durable tumor control and complete regression in 40% of cases when combined with enzalutamide, highlighting its potential in overcoming treatment resistance.
Clinical Implications: The novel mechanism of ATNM-400 offers a promising alternative for patients with metastatic castration-resistant prostate cancer (mCRPC) who have limited options due to low or absent PSMA expression, addressing a significant unmet clinical need.
Future Directions: Actinium plans to explore ATNM-400's applications beyond prostate cancer, including non-small cell lung cancer (NSCLC), where it has shown potential to overcome resistance to existing therapies, further expanding its therapeutic impact.